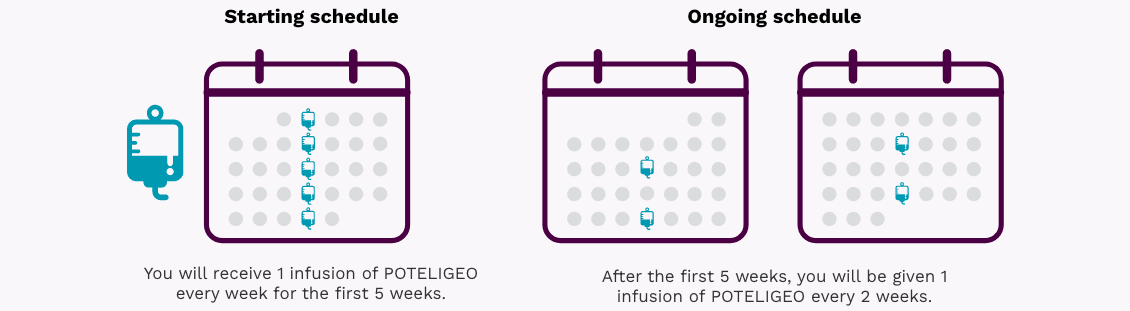
What is the most important information I should know about
POTELIGEO?
POTELIGEO may cause serious side effects that can be severe or life-threatening
including
skin problems, infusion reactions, infections, autoimmune problems, and complications
from stem cell transplant.
Call or see your healthcare provider right away if you develop any symptoms of the
following
problems or if these symptoms get worse:
-
Skin problems: Signs and symptoms of skin
reactions may include skin
pain,
itching, skin blistering or peeling, rash, painful sores or ulcers in your
mouth, nose,
throat
or genital area.
-
Infusion reactions: Signs and symptoms of
infusion reactions may
include chills or
shaking, redness on your face (flushing), itching or rash, shortness of
breath, coughing
or wheezing,
dizziness, feeling like passing out, tiredness, fever.
-
Infections: Signs and symptoms of
infection may include fever, sweats
or chills,
nausea, flu-like symptoms, sore throat or difficulty swallowing, shortness
of breath,
diarrhea or
stomach pain, cough.
-
Autoimmune problems: Some people receiving
POTELIGEO may develop
autoimmune problems,
and some people who already have an autoimmune disease may get worse during
treatment
with POTELIGEO.
-
Complications of stem cell transplant:
Patients who receive a stem cell
transplant
using donor stem cells (allogeneic) after treatment with POTELIGEO may
experience
complications that
can be severe and lead to death. Your healthcare provider will monitor you
for signs of
complications
if you have an allogeneic stem cell transplant.
What are the most common side effects of POTELIGEO?
The most common side effects of POTELIGEO include rash, tiredness, diarrhea, muscle
and bone pain, and upper respiratory tract infection.
Before starting POTELIGEO treatment, tell your doctor about all your medical
conditions,
including whether you:
-
have had a severe skin reaction after receiving POTELIGEO
-
have had an infusion reaction during or after receiving POTELIGEO
-
have or have had liver problems including hepatitis B (HBV) infection
-
have a history of autoimmune problems
-
have undergone or plan to have a stem cell transplant, using cells from a
donor
-
have lung or breathing problems
-
are pregnant or plan to become pregnant
- It is not known
if POTELIGEO will harm your unborn baby
-
are breastfeeding or plan to breastfeed
- Talk to your
healthcare provider about the best way to feed your baby during
treatment with POTELIGEO
Tell your doctor about all the medicines you take, including prescription and
over-the-counter
medicines, vitamins, and herbal supplements.
You are encouraged to report suspected adverse reactions to Kyowa Kirin, Inc. at
1-844-768-3544 or FDA
at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see full Prescribing Information as well as Patient Information.